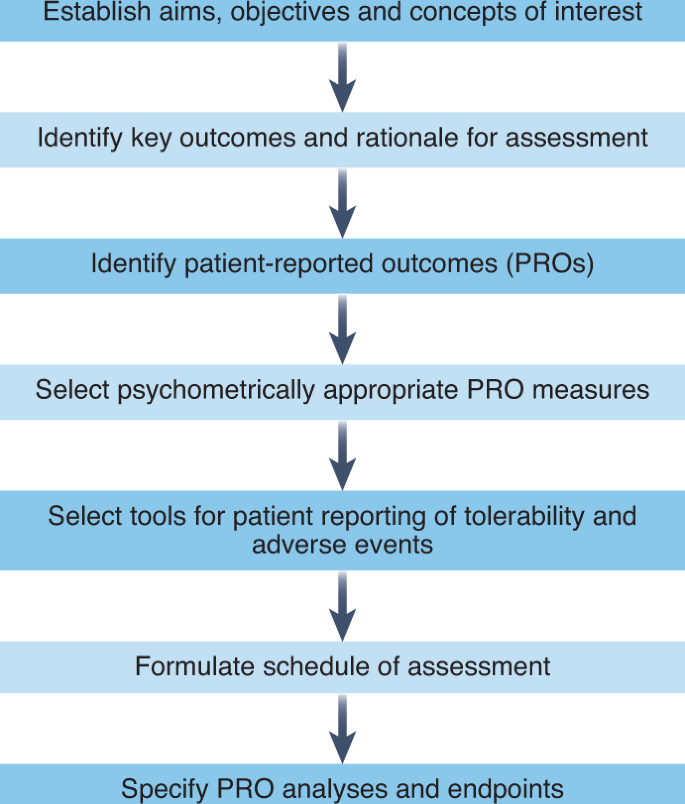
Patient-reported outcomes: A new era in clinical research Deshpande PR, Rajan S, Sudeepthi B L, Abdul Nazir C P - Perspect Clin Res

Patient-reported outcomes: A new era in clinical research Deshpande PR, Rajan S, Sudeepthi B L, Abdul Nazir C P - Perspect Clin Res
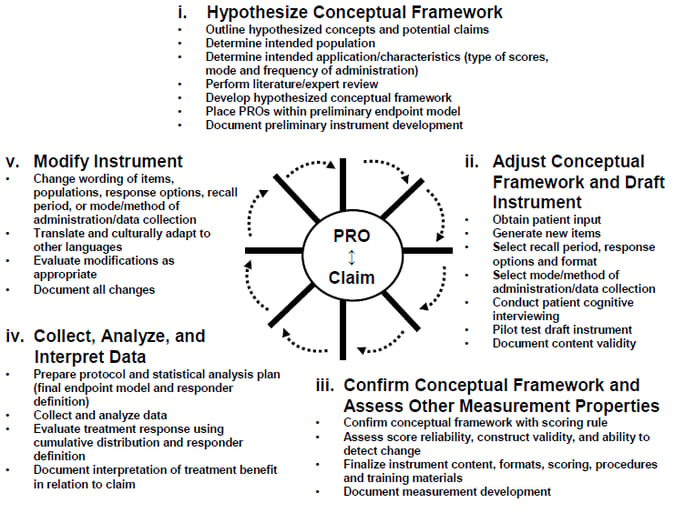
Development and Content Validation of a Clinically Meaningful Patient-Reported Outcome (PRO) for Scalp Hair Assessment

Patient-reported outcomes: A new era in clinical research Deshpande PR, Rajan S, Sudeepthi B L, Abdul Nazir C P - Perspect Clin Res
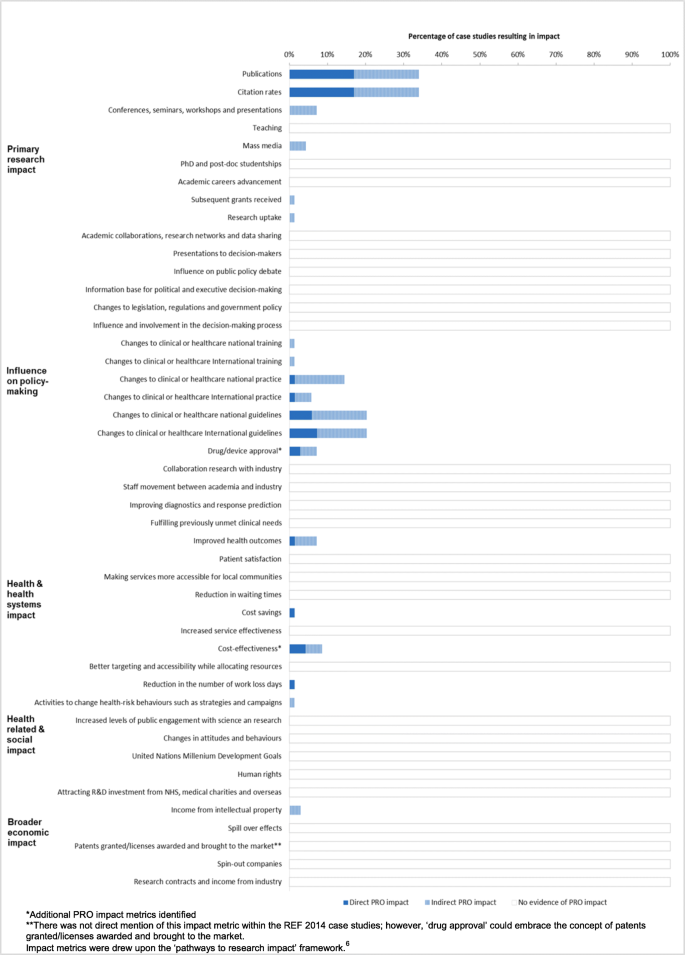
The impact of patient-reported outcome (PRO) data from clinical trials: a systematic review and critical analysis | Health and Quality of Life Outcomes | Full Text
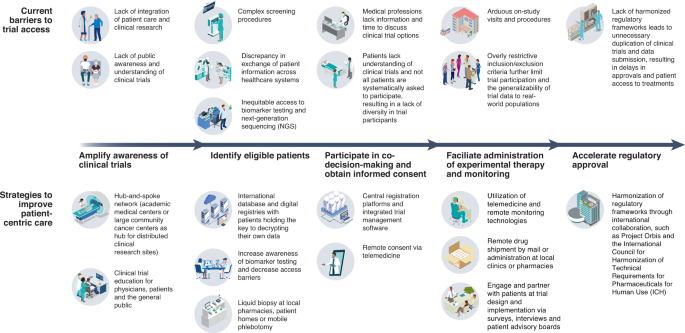
Reimagining patient-centric cancer clinical trials: a multi-stakeholder international coalition | Nature Medicine
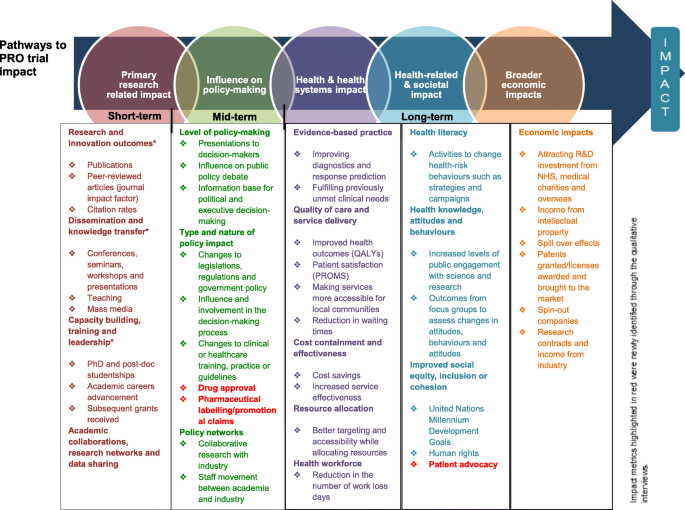
The impact of patient-reported outcome data from clinical trials: perspectives from international stakeholders | Journal of Patient-Reported Outcomes | Full Text
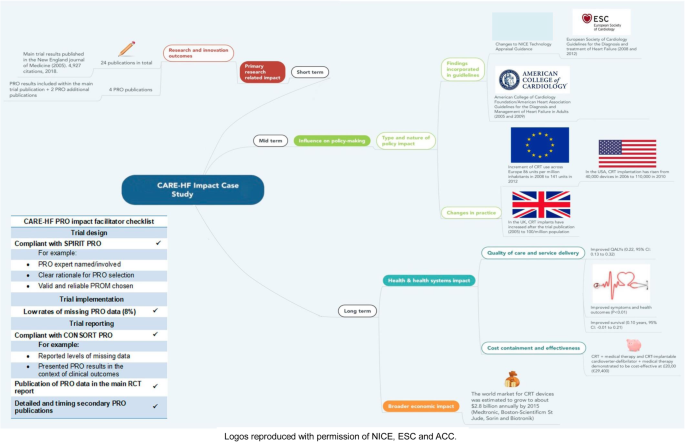
The impact of patient-reported outcome (PRO) data from clinical trials: a systematic review and critical analysis | Health and Quality of Life Outcomes | Full Text

Content Validity—Establishing and Reporting the Evidence in Newly Developed Patient-Reported Outcomes (PRO) Instruments for Medical Product Evaluation: ISPOR PRO Good Research Practices Task Force Report: Part 2—Assessing Respondent Understanding ...
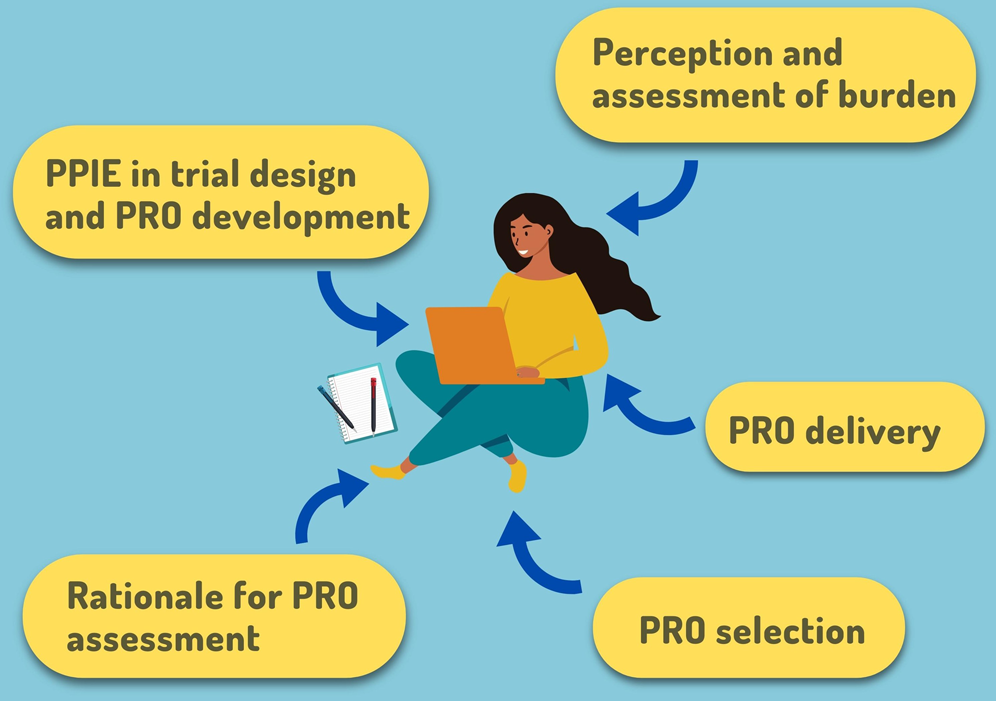
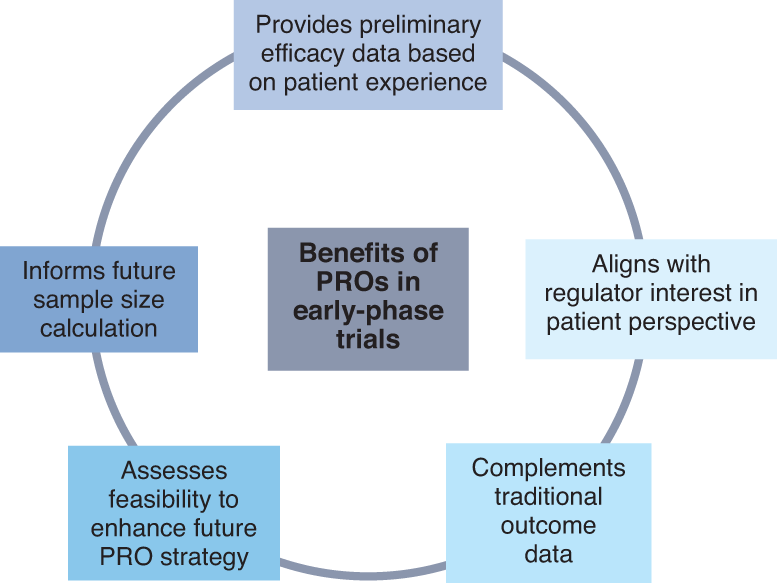
![PDF] Patient-reported outcomes: A new era in clinical research | Semantic Scholar PDF] Patient-reported outcomes: A new era in clinical research | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/78914c352ad5c90cf5f938630642000756bf716b/5-Figure4-1.png)


![PDF] Patient-reported outcomes: A new era in clinical research | Semantic Scholar PDF] Patient-reported outcomes: A new era in clinical research | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/78914c352ad5c90cf5f938630642000756bf716b/3-Figure3-1.png)